My Blog
Tips, Resources, and Strategies for High School Science

Calculating the Equilibrium Constant Keq
Hey there! In this blog, we will walk through the steps for calculating the equilibrium constant Keq. We’ll use the following question as an example.
Calculate the equilibrium constant Keq, for the reaction between nitrogen monoxide and oxygen to form nitrogen dioxide if the concentrations for NO, O2, and NO2 are 0.10 M, 0.10 M, and 0.20 M, respectively.
STEP 1: WRITE THE BALANCED CHEMICAL EQUATION
The first step for calculating the equilibrium constant Keq, we need to write down the balanced chemical equation for the reaction. For the given reaction, the balanced chemical equation is:
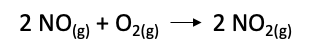
STEP 2: WRITE THE EXPRESSION FOR THE EQUILIBRIUM CONSTANT Keq
This is basically the products’ concentrations divided by the reactants’ concentrations, with each one raised to the power of their stoichiometric coefficients. So for our reaction, the expression of the equilibrium constant, Keq, is:
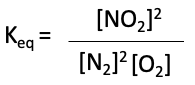
where [NO2] is the equilibrium concentration of the product, and [NO] and [O2] are the equilibrium concentrations of the reactants.
STEP 3: CALCULATE THE EQUILIBRIUM CONSTANT, Keq
Finally, we need to calculate Keq. We can do this by finding out the equilibrium concentrations of the products and reactants. We can plug the concentration values for NO, O2, and NO2 into our expression for Keq.
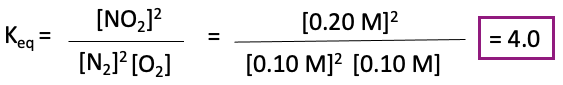
Therefore, the equilibrium constant, Keq, for the reaction 2 NO(g) + O2(g) –> 2 NO2(g) is 4.0.
And that’s it! By following these steps, you can find the equilibrium expression, Keq, for any chemical reaction. This can help you understand how far the reaction has gone towards the products or the reactants, which is useful in chemistry.
For more practice, watch this video.
All Rights Reserved 2023 - (C) TheScienceMentor.com -TM | Terms & Conditions | Privacy Policy | Disclaimers
